NGS assay for detecting bladder cancer biomarkers in urine for research purposes
GALEAS™ Bladder (RUO)
A non-invasive NGS enrichment assay for identifying bladder cancer biomarkers from a urine sample.
GALEAS Bladder is for research use only and not for use in diagnostic procedures outside of the UK. Our UKCA marked product can be found here: GALEAS Bladder (UKCA)| Nonacus
GALEAS Bladder leverages ultra-sensitive, targeted next generation sequencing (NGS) chemistry to interrogate somatic mutations in 23 genes associated with bladder cancer. Using genomic DNA (gDNA) extracted from urine samples, it offers a non-invasive approach to research studies into the genomics of bladder cancer.
Key Features
- Covers 23 genes associated with 96% of bladder cancer cases.
- Enables research into multiple stages of bladder cancer.
- Designed for investigation of different bladder cancer classifications including NMIBC and MIBC.
- Optimiszed from sample to data for research laboratory efficiency.
- Can be run in any NGS capable laboratory and automated and scaled according to throughput.
GALEAS Bladder workflow
Service laboratory dispatches bar coded GALEAS urine collection device for sample collection
Returned urine sample is centrifuged and gDNA extracted from cell pellet using Bead Xtract urine gDNA extraction kit
NGS libraries are prepared with the GALEAS Bladder kit and sequenced. 5M reads (2 x 150bp PE reads)
FASTQ files are uploaded to secure cloud-based GALEAS analysis platform
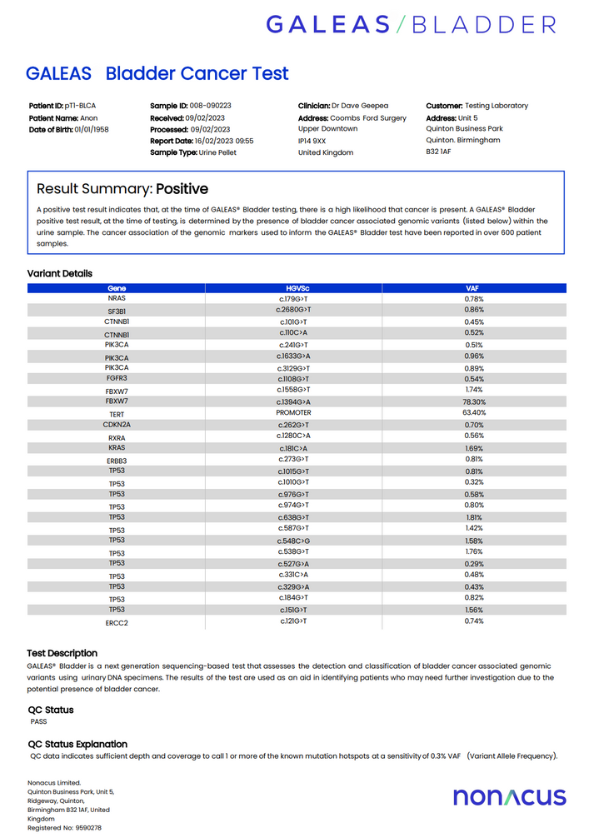
Results are analysed and reports generated for research purposes
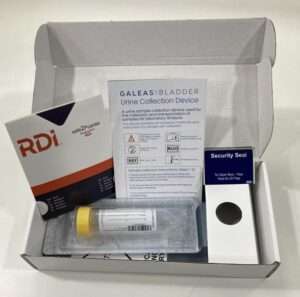
A simple, intuitive cardboard collection device provides a convenient method for urine sample collection.
The urine sample is returned to the lab in a 50ml Falcon tube containing our proprietary preserver validated with our DNA extraction kit and panels to ensure optimal performance.
The tube is barcoded to provide full traceability.
GALEAS Bladder has been evaluated in research studies for its analytical performance in detecting tumor derived DNA
Using 710 urine samples collected from three UK cohorts, GALEAS Bladder demonstrated the ability to detect somatic mutations reported in over 96% of bladder cancer cases in published literature.
| Sensitivity | Specificity | |
|---|---|---|
| pTa | 89% | 86% |
| T1 | 97% | 86% |
| T2+ | 92% | 86% |
| G1 | 78% | 86% |
| G2 | 93% | 86% |
| G3 | 96% | 86% |
| NMIBC | 92% | 86% |
| MIBC | 92% | 86% |
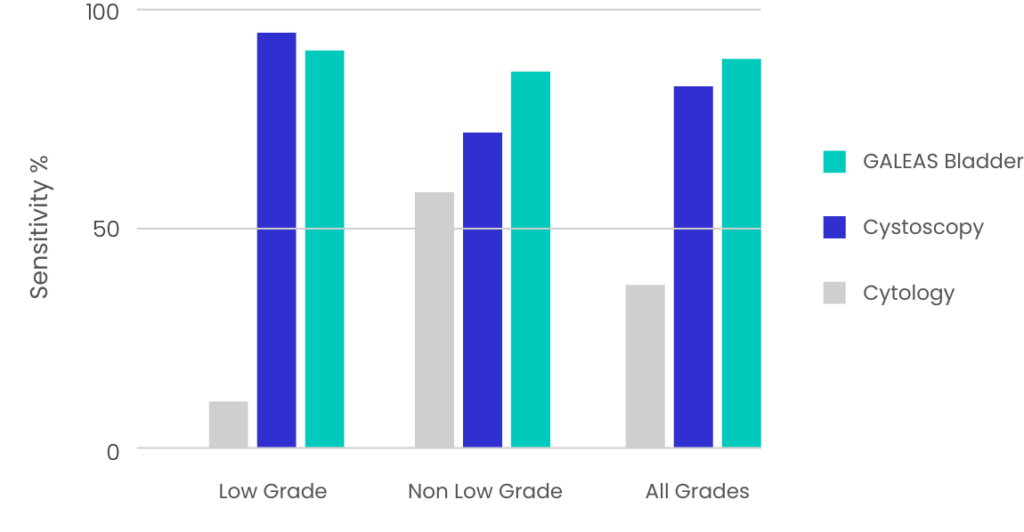
Figure 1: GALEAS Bladder performance across all stages of bladder cancer and compared to reported sensitivity of cytology and cystoscopy.
Why choose GALEAS Bladder?
Simple end to end workflow for easy implementation
From ready to ship urine collection devices through to secure cloud-based reporting the process is optimized to maximize efficiencies.
Optimized reagents ensure reliability of test results
All reagents associated with the GALEAS Bladder workflow have been developed and optimized to ensure consistent and reliable results.
Flexible throughput offers scalability
By covering a wide range of markers, GALEAS Bladder can be used for patient monitoring, relapse, MRD surveillance and companion diagnostics as well as hematuria triage.
References
1. Ward DG, Baxter L, Ott S, Gordon NS, Wang J, Patel P, et al. Highly sensitive and specific detection of bladder cancer via targeted ultra-deep sequencing of urinary DNA. European Urology Oncology. 2023 Feb;6(1):67-75.