Tumour Mutational Burden (TMB) and Microsatellite Instability (MSI) as markers for immunotherapy response
Published December 22, 2021 | Reviewed by Victoria Simms on April, 22, 2024
The promise of Immune-checkpoint inhibitor therapies
Immune-checkpoint inhibitor (ICI) therapies have heralded promise of a superior drug response and significantly increased survival for a small proportion of cancer patients across a range of cancers. Their success lies in the inhibition of biological mechanisms that prevent immune cells from attacking other cells. The challenge for immuno-oncology is to identify those patients most likely to benefit.
Role of ICIs in cancer immunotherapy
ICIs are one of the most rapidly growing categories of immune‐related agents developed for the treatment of cancer. They are monoclonal antibodies designed to inhibit specific immune checkpoints (protein regulators of the immune system). Two of these checkpoint targets on T-cells are:
- programmed cell death protein 1 (PD‐1) and
- cytotoxic T‐lymphocyte-associated protein 4 (CTLA‐4)
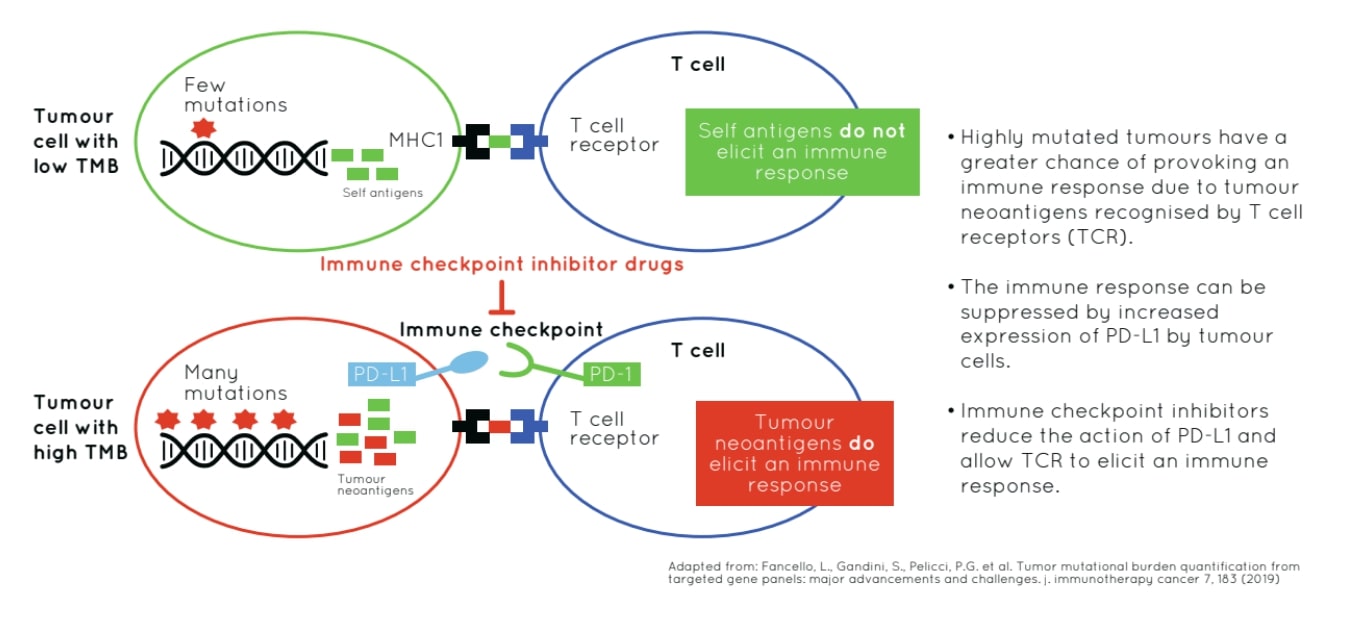
Programmed cell death-ligand 1 (PD-L1), is expressed on tumour and T-cells and is considered a target, as this molecule binds PD-1 effectively reducing T-cell response. Mutations arising in tumour cells may act as neoantigens recognized by the immune system. The aim of ICI treatments is to boost the immune response to attack tumor cells presenting with high neoantigen load. 1
PD-L1 expression using immunohistochemistry (IHC) techniques has been identified as a predictive biomarker for immuno-oncology in multiple cancer types. However, this method has failed to detect a significant proportion of patients likely to respond to ICI therapies.2 There has been an urgent need to find further biomarkers and this blog article discusses two approaches currently gaining momentum.
Tumour Mutational Burden (TMB) as a biomarker for predicting response to ICI treatments
TMB, defined as the total number of different somatic alterations or mutations in a tumour, differs across tumour types. TMB has been identified as a potential biomarker for predicting response to ICI treatments because neoantigens that can arise from a subset of tumour mutations, are identified by the immune system and targeted for destruction. Increased somatic mutations potentially equals an increased neoantigen load.
Estimation of TMB is a useful approach to ascertain the neoantigen level in a patient's tumour. 3 Clinical studies demonstrate that, in patients receiving ICI therapies, higher TMB results in significantly improved survival for multiple cancer types including colon, melanoma, and non-small cell lung cancer (NSCLC). For example, recent clinical trial data indicated that for NSCLC, high TMB was associated with significantly longer progression free survival in patients receiving immunotherapy drugs supporting the use of TMB as a predictive biomarker for treatment with ICIs.4
Calculating TMB with whole exome sequencing
Initially, calculations of TMB focused on data derived from whole exome sequencing (WES). In this approach only nonsynonymous single base changes, those resulting in the substitution of a different amino acid, are typically counted. The assumption being that there is a positive correlation between protein coding changes in the tumor genome and neoantigen load.2
Targeted NGS panels for measuring TMB in the clinic
Whilst whole exome sequencing shows encouraging results, it remains too costly for routine clinical use. Consequently, targeted next generation sequencing (NGS) panels to determine TMB are beginning to be introduced for immuno-oncology. To circumvent issues arising from the use of smaller genomic regions, targeted panels include synonymous as well as non-synonymous single base changes and short insertions and deletions (INDELs). The premise here is that the accumulation of numerous synonymous variants suggests an increased level of mutagenesis and their inclusion improves the analytical sensitivity of the TMB calculation.2
Research by Buchhalter et al. has demonstrated the importance of panel size in the accuracy of the TMB calculation.5 Specifically, they reported large confidence intervals when TMB calculations used small, targeted panels <1Mb. However, panels between 1.5 Mb and 3 Mb revealed significantly smaller confidence intervals indicating their relevance in the TMB calculations.5
"Optimizing the cost-benefit ratio, our data suggest that panels between 1.5Mb and 3Mb are ideally suited to estimate TMB with small confidence intervals."
Buchhalter et al., 2018.
Microsatellite Instability (MSI)
Microsatellite instability (MSI), characterized by hypermutability, is associated with defective DNA mismatch repair (MMR) systems. Microsatellites, short fragments of repeat DNA sequences, are prone to the accumulation of errors. When the MMR system, that exists to repair these errors arising from normal DNA replication, is impaired this causes a significant upregulation in the mutation rate.6
Comprehensive genomic profiling (CGP) with pan-cancer panels
CGP performed by targeted NGS panels, aims to detect all types of genomic alterations including single nucleotide variants (SNV), small and large indels, gene rearrangements and copy number variants (CNV) in relevant cancer-associated genes as well as large genomic signatures such as MSI, loss of heterozygosity (LOH), and TMB. By combining MSI, LOH and TMB, the final TMB calculation is comparable to TMB derived from WES.2,8
Recent research in colorectal cancer has further highlighted the advantages of using NGS panels to determine TMB and MSI status. In their study, Xiao et al. (2021), found an NGS panel approach was highly concordant with IHC and concluded that combining MSI and TMB may be better suited to identifying patients with a more active immune response.9
"A strategy combining MSI and TMB determination is of interest in diagnostics as it provides additional information for comprehensive molecular characterization of colorectal cancer patients and this may indicate their suitability for ICI treatment."
Xiao et al., 2021.
GALEAS Tumor enables reliable and accurate TMB and MSI estimation. Using comprehensive genomic profiling will help inform immunotherapy treatment options for your patients potentially saving precious time and significant cost.
References
- Galuppini F, et al. (2019) Tumor mutation burden: from comprehensive mutational screening to the clinic. Cancer cell international;19(1):1-10.
- Klempner S, et al. (2020) Tumor Mutational Burden as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors: A Review of Current Evidence. Oncologist: Jan;25(1):e147-e159.
- Chan TA, et al. (2019) Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Annals of Oncology. ;30(1):44-56.
- Bai R, et al. (2020) Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res 8, 34.
- Buchhalter I, et al. (2019) Size matters: Dissecting key parameters for panel‐based tumor mutational burden analysis IJC 144, 848-858.
- Eso Y, et al. (2020) Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol 55, 15-26.
- Kawakami H, et al. (2015) Microsatellite instability testing and its role in the management of colorectal cancer. Current treatment options in oncology. ;16(7):1-15.
- Endris V, et al. (2019) Measurement of tumor mutational burden (TMB) in routine molecular diagnostics: in silico and real‐life analysis of three larger gene panels. IJC 144, 2303-2312.
- Xiao J, et al. (2021) A next-generation sequencing-based strategy combining microsatellite instability and tumor mutation burden for comprehensive molecular diagnosis of advanced colorectal cancer. BMC Cancer 21, 282.