Fast and flexible
Cell3 Xtract has a simple and flexible protocol that requires no specialist equipment and, with just 90 minutes of processing time, will speed up your cfDNA extractions.
Maximize sample input
Whether you are working with 1 ml or 10 ml you can extract all cfDNA in one extraction. With a small elution volume of just 35 µl you can avoid the need for a DNA concentration step.
Supports multiple biological sample types
Isolate cfDNA from a wide range of sample types: plasma, cerebrospinal fluid (CSF), saliva and amniotic fluid.
Accurate quantification
Efficient cfDNA extraction without carrier RNA ensures accurate cfDNA quantification for sensitive downstream applications like NGS.
Optimizing cell-free DNA extraction from plasma
The utility of cfDNA in both translational research and diagnostic settings has increased dramatically since its discovery. These short fragment sizes of ~160 bp are typically found in low quantities of 1-100 ng/ml in plasma. It is therefore critical that sufficient good quality cfDNA is extracted from biological samples for downstream applications like NGS and qPCR.
To address this need, Cell3 Xtract has been developed as a fast, accurate and flexible cell-free DNA extraction kit designed to optimize extraction and maximize cfDNA yield.
Large volume cell-free DNA extraction
Cell3 Xtract has a simple and flexible workflow which allows 1-10 ml of a biological sample to be processed within 90 minutes.
The flexible input volume allows for increased recovery and concentration of cfDNA from an entire sample, avoiding the necessity for multiple 1 ml extractions. No specialist equipment such as magnets or vacuum manifolds are required.
Obtain higher cfDNA concentration
With a small elution volume of just 35 µl, regardless of your starting volume, you can avoid the need for a DNA vacuum concentration step. This means you can add more cfDNA to your downstream applications including ddPCR and NGS enabling more sensitive detection of variants.


Accurate quantification of cfDNA
Accurate quantification of cell-free DNA extraction is important for downstream applications, particularly Next Generation Sequencing (NGS) where input amounts for assays can be critical. Many companies use carrier RNA to improve cfDNA extraction which can lead to an overestimate of cfDNA quantification and compromise the effectiveness of NGS library preparation.
The Cell3 Xtract cfDNA extraction kit does not require carrier RNA to optimize cfDNA isolation enabling accurate quantification of cfDNA and efficient library preparation.
Superior technical performance
We compared Cell3 Xtract to spin column and bead-based cfDNA extraction kits from four other companies.
Using cfDNA extracted from 1 ml plasma from a pregnant woman carrying a male fetus, qPCR, DNA concentration and fragment size analysis showed that, in the majority of cases, Cell3 Xtract outperformed other manufacturers.
Comparison to spin-column based kits
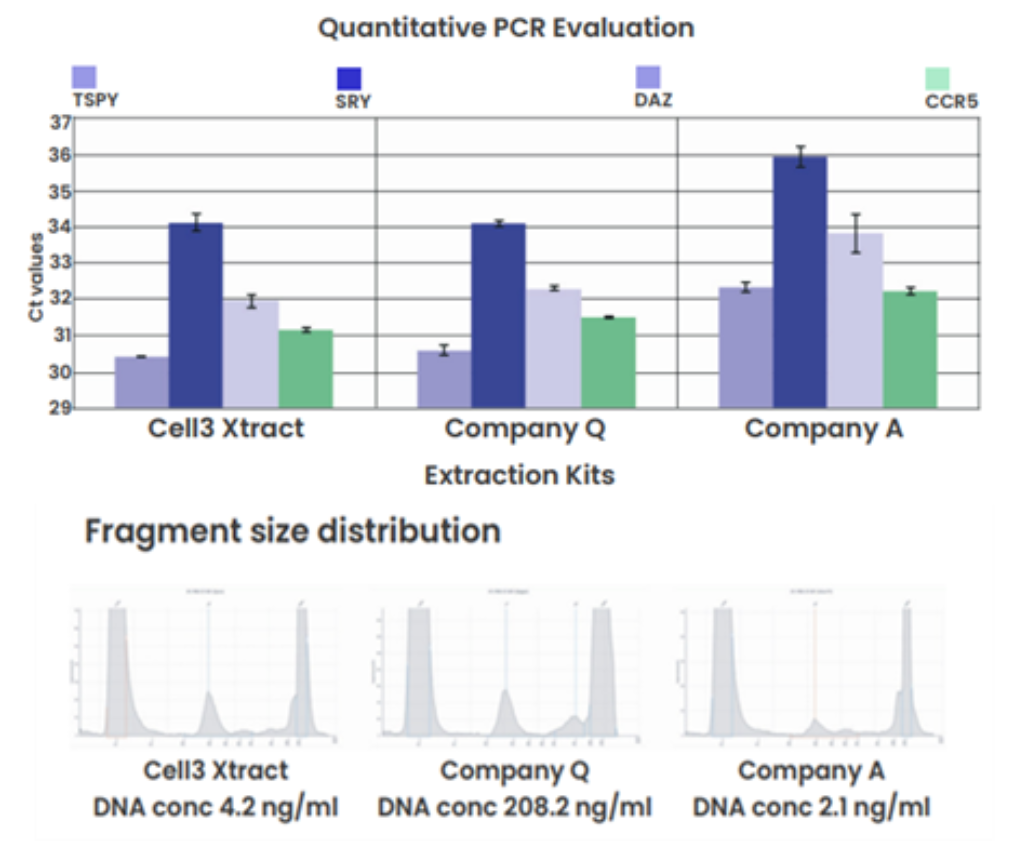
Figure 1: Column-based cfDNA extraction kits: qPCR data revealed that the Cell3 Xtract kit performed on par with the Company Q kit and outperformed the Company A kit. However, the use of carrier RNA in the Company Q kit resulted in a 50-fold higher DNA concentration measurement and the appearance of a high molecular weight peak in the fragment analysis electropherogram.
Comparison to bead-based kits
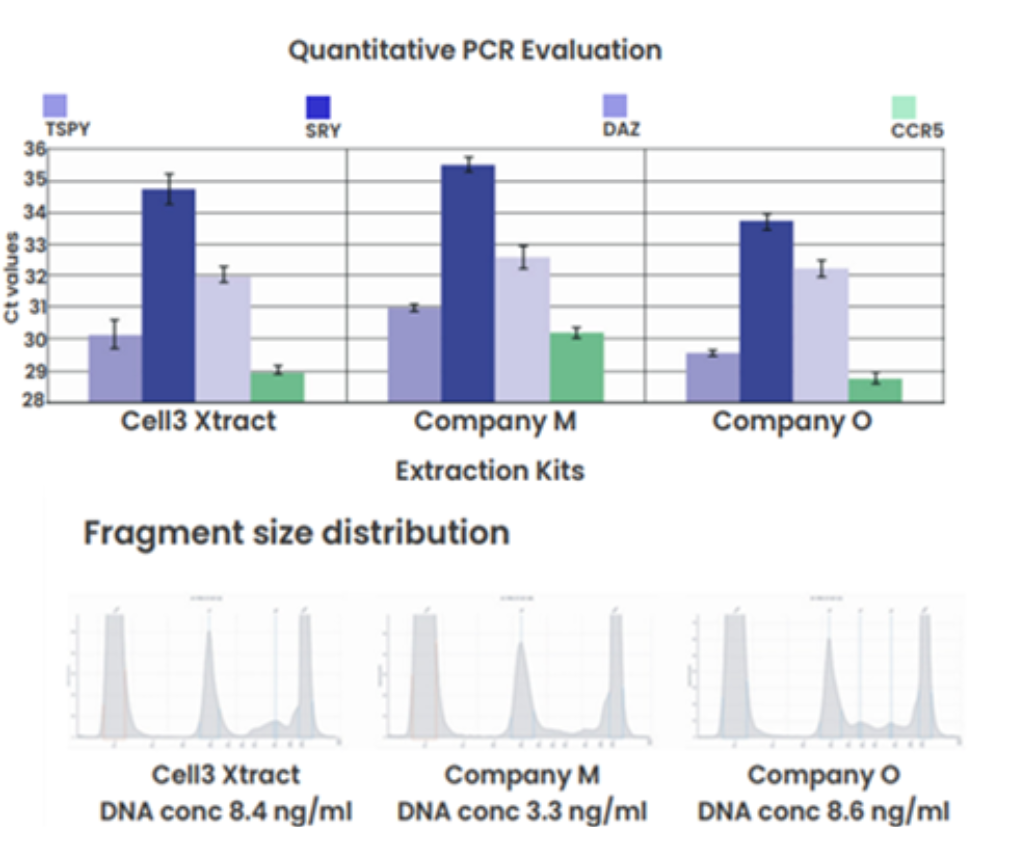
Figure 2: Bead-based cfDNA extraction kits: qPCR and DNA concentration data showed that the Cell3 Xtract kit performed better than the Company M kit and on par with the Company O kit.
For Research Use Only. Not for use in diagnostic procedures.
See our customer publications
| Product | Catalog No. |
| Cell3 Xtract 16 Samples | PRE_EXT_C3X_16 |
| Cell3 Xtract 48 samples | PRE_EXT_C3X_48 |
How should I store the plasma sample prior to cell-free DNA extraction?
Plasma can be stored at -20°C for long term storage, and we recommend using DNA LoBind tubes for this purpose. Prior to cell-free DNA extraction, the plasma sample should be thawed at room temperature or in a heat block or water bath at 37°C.
How should I store my eluted cell-free DNA sample?
Eluted DNA can be used immediately for downstream applications or stored at ≤ -20°C. We recommend using DNA LoBind tubes for storing DNA samples.
Do I need any specialist equipment when using the Cell3 Xtract kit?
No specialist equipment such as magnets or vacuum manifolds are required. You will need the following which is standard in most blood handling laboratories: water bath or heat block (55°C), microcentrifuge (capable of accommodating 1.5-2 ml tubes) and a swing bucket centrifuge (capable of accommodating 15-50 ml tubes).
What spin speeds should I use to isolate the plasma prior to using a cell-free DNA extraction kit?
We recommend using a two-step centrifugation process to isolate the plasma, an initial spin speed of 2000 g for 10 minutes to isolate the plasma fraction from the red and white blood cells, then a second high spin of minimum 10,000 g for 10 minutes to pellet and remove intact cells and cellular debris from the plasma fraction.
How can I remove any precipitates from the buffers in my extraction kit?
During shipment or storage in cool ambient conditions, precipitates may form in some buffers. Dissolve such deposits by warming the solution at 37°C and gently shaking.
