Written by Victoria Simms May 2023. Reviewed by Celina Whalley April 2024

May marks Bladder Cancer Awareness Month, four weeks dedicated to raising awareness of this disease, its symptoms, and the current realities for those impacted by bladder cancer both directly and indirectly.
As we continue to implement GALEAS™ Bladder, at Nonacus we want to use Bladder Cancer Awareness Month to raise awareness of the disease and emphasize the importance of discussions around bladder cancer and how to recognize early symptoms.
UK bladder cancer statistics1
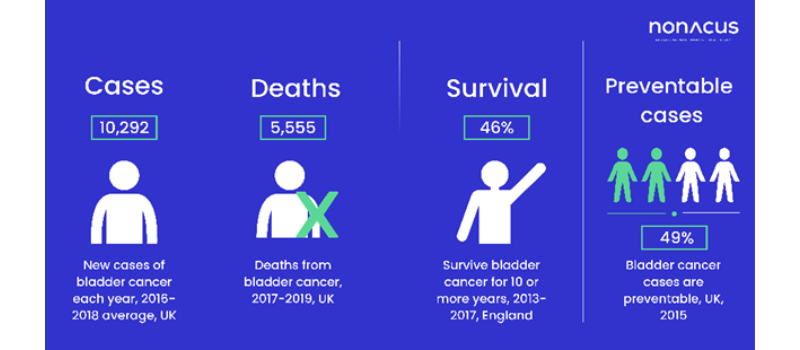
Whilst bladder cancer survival rates in England are above the European average, there is a strong link between early detection and improved patient outcomes
Statistically in the UK, bladder cancer is the 11th most common cancer with around 10,300 new cases annually and whilst survival rates in England are above the European average, there is a strong link between early detection and improved patient outcomes.1
So, what is the most common visible symptom of bladder cancer?
The leading indicator is hematuria (blood in the urine) with around 80 out of 100 sufferers experiencing this symptom.3 After presenting to the GP with hematuria, patients will be referred for several tests to determine if bladder cancer is present, including a flexible cystoscopy; an invasive procedure that requires the insertion of a camera into the bladder.
Whilst this is currently an essential step for the triage of patients with hematuria, in the UK alone, over 110,000 flexible cystoscopies are carried out each year at a cost of millions to the NHS and the procedure causes significant discomfort to patients.
"Typically, patients undergoing cystoscopy for bladder cancer detection often express concerns about the invasive nature of the procedure. They may worry about potential pain or discomfort during the examination, as well as any potential complications that could arise. Many individuals also feel anxious or embarrassed about the lack of privacy during the procedure, as it involves the insertion of a cystoscope into the urethra." states Lydia Makaroff, CEO of Fight Bladder Cancer
Furthermore, 88% of these cystoscopies turn out to be unnecessary as the patient has no abnormality or malignancy.4 There is therefore a clear need for a non-invasive, highly accurate and sensitive test to improve bladder cancer management for patients; by reducing the number of cystoscopies necessary for hematuria triage and for monitoring patients with a diagnosis of bladder cancer.
To offer a credible alternative, it's essential to note that flexible cystoscopy typically has a sensitivity and specificity of about 85-90% for detecting bladder tumors.5 Consequently, any DNA-based assay proposed as an alternative must aim to achieve similar levels of accuracy, if not match them.
At Nonacus, we have developed GALEAS™ Bladder, a comprehensive biomarker test that can deliver the specificity and sensitivity equivalent to cystoscopy, for all bladder cancer grades, from a simple urine sample. GALEAS Bladder offers a streamlined sample-to-report solution for doctors, clinics, service laboratories and patients offering the potential for use across the whole bladder cancer care pathway.

“A recent breakthrough in diagnostics could make it easier to detect patients early”
Developed in partnership with researchers at the University of Birmingham, UK, the test leverages ultra-sensitive targeted Next Generation Sequencing (NGS) to interrogate the key somatic mutations found across all grades and stages of bladder cancer.
The solution has sensitivity and specificity equivalent to cystoscopy across all grades of bladder cancer, including non-muscle invasive and muscle invasive bladder cancer. By delivering sensitivity of >90%, GALEAS Bladder allows urologists to make informed, safe decisions about which patients need further investigation and which don't. Thereby, reducing the burden on healthcare systems and allowing for the prioritized referral of those who may have bladder cancer.
As we continue to implement GALEAS Bladder, we want to use this month to raise awareness of the disease and emphasize the importance of discussions around bladder cancer and knowledge of early symptoms. For further information on this, please visit www.fightbladdercancer.co.uk.
Want to find out the development of our novel GALEAS Bladder test? Watch on demand our GenomeWeb webinar where Prof. Richard Bryan and Dr. Douglas Ward speak about how GALEAS Bladder was validated using urine samples from three UK clinical cohorts
Author's note: Fight Bladder Cancer is an independent registered charity that bears no affiliation to Nonacus or GALEAS® Bladder. They do not endorse GALEAS® Bladder specifically and always recommend referral to your GP if you have any concerns about Bladder Cancer. (Approved by Lydia Makaroff)
References:
- Bladder cancer statistics, Cancer Research UK, Accessed April 15, 2024
- Bladder Cancer: Statistics | Cancer.Net, Accessed April 15, 2024
- Symptoms of bladder cancer, Cancer Research UK, Accessed April 15, 2024
- Kelly JD, Fawcett DP, Goldberg LC. Assessment and management of non-visible haematuria in primary care. BMJ. 2009;338.
- Zheng C, Lv Y, Zhong Q, Wang R, Jiang Q. Narrow band imaging diagnosis of bladder cancer: systematic review and meta‐analysis. BJU international. 2012;110(11b):E680-7.