More than 20 NHS Trusts now plan to join the GALEAS Bladder service evaluation
Our ‘GALEAS Bladder – adoption in practice’ event took place last week, alongside the BAUS Oncology event, together generating a huge amount of excitement and interest in our NHS service evaluation of GALEAS Bladder, a non-invasive urine test for identification and monitoring of bladder cancer that delivers results equivalent to a cystoscopy.
Co-developed by Professor Rik Bryan and Dr Doug Ward from the University of Birmingham, and funded by Cancer Research UK, the GALEAS Bladder test is already being evaluated by seven NHS Trusts to see how the results compare to a cystoscopy in a real-world clinical setting. Following the event last week, the numbers of interested Trusts grew to over 20, all keen to discover how this simple urine test could transform their cancer pathway.
GALEAS Bladder – adoption in practice event
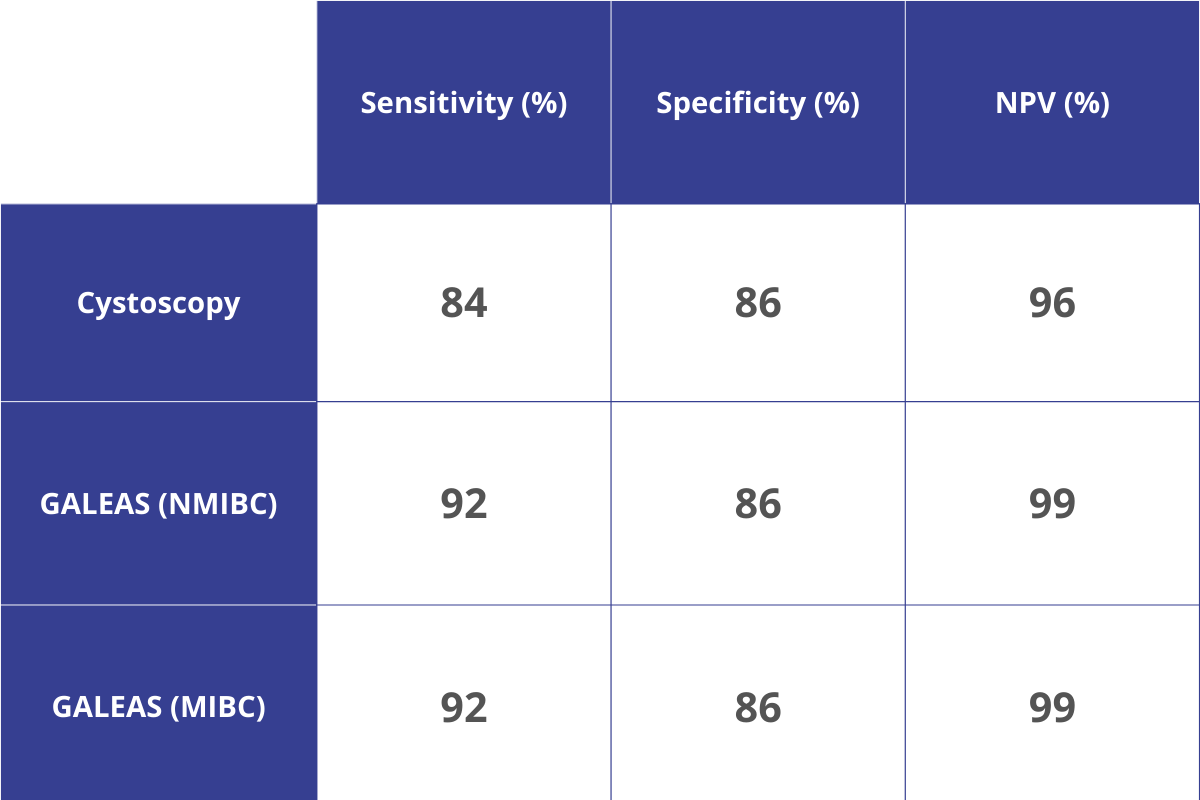
At our special dinner event, Professor Rik Bryan was joined by two of our NHS service evaluation participants, Uwais Mufti, Consultant Urologist from Leeds and John Piedad, Specialist Registrar/Clinical Teaching Fellow in Urology, Royal Berkshire Hospital, demonstrating that the sensitivity and specificity delivered by the GALEAS Bladder test is proving to be as previous studies. All three discussed exciting future use cases for the test, including using the test in a surveillance setting and for upper tract cancers.
David Hutchinson, Chief Operating Officer at Berkeley Genetics and attendee at the event, said: “Hearing the data presented about GALEAS Bladder and the impact that this will have on bladder cancer patients and professionals, will revolutionise the way in which care is given. We are excited to be on the cusp of seeing the way in which this can deliver actionable insights and ensure a smooth and effective route to the right treatment for so many at risk patients.”
What are the aims of the GALEAS Bladder NHS service evaluation?
- Demonstrate the ability of safely using the GALEAS Bladder service as an effective tool for the haematuria triage of patients, reviewing the sensitivity, specificity and negative predicted value (NPV) of the GALEAS Bladder test in practice
- Support clinical adoption into trust pathways should this be a desired approach may be helpful to guide treatment options and also likelihood of MIBC or NMIB
- To build clinical experience of the GALEAS Bladder test in the real-world clinical environment.
How does it work?
- Informed Genomics provides complimentary GALEAS Bladder testing kits to the NHS Trust, supported by face-to face training and materials to help onboard the teams and provide information to patients.
- Patients undergoing a cystoscopy in a triage setting also provide a urine sample for the GALEAS Bladder test.
- The sample is sent to the Informed Genomics UK laboratory for testing.
- A patient report is created indicating if cancer is present or not and if so a list of the variants found. These in turn may be helpful to guide treatment options and also likelihood of MIBC or NMIBC.
- Participating Trusts join a working group, with regular calls to discuss progress, share best practice and help resolve any challenges.
If you would like to find out more about the NHS service evaluation, please contact us today.