Validated for cell-free and genomic DNA
Next generation sequencing (NGS) enrichment developed for and validated on cell-free DNA as well as gDNA for prenatal applications like non-invasive prenatal diagnosis.
Simple and rapid design process
Our online panel design tool means you can design and optimize a panel in minutes and our novel rapid manufacturing process means minimal time between design and delivery.
Confidently detect fetal fraction in maternal blood
QC validated to guarantee uniformity of coverage which, combined with error suppression technology, ensures confident detection of even the lowest fetal fraction.
Optimized for large and small panels
Optimized to deliver efficient target capture and unrivalled on-target performance regardless of panel size from 1-1000 genes.
Non-invasive prenatal testing (NIPT)
Cell-free DNA (cfDNA) are degraded DNA fragments released into the bloodstream through a natural process of cell death. During pregnancy, the mother’s blood contains cfDNA from her own tissue and from the fetus (cffDNA) via the placenta. Sequence analysis of cfDNA from a sample of maternal blood can therefore provide genetic information about the fetus and inform clinical decisions without the need for invasive techniques like amniocentesis. However, as only between 2-20% of the total cell-free DNA in maternal blood is fetal (the fetal fraction), its analysis requires ultra-sensitive sequencing methods capable of accurately detecting genetic variants in samples with even the lowest of fetal fractions.1,2
Targeted NGS/resequencing panels for non-invasive prenatal diagnosis (NIPD) or NIPT
Targeted sequencing, through the use of custom designed NGS panels, offers a cost-effective method for the ultra-sensitive sequencing required for non-invasive prenatal genetics. Without the data burden of whole-genome sequencing (WGS) or whole-exome sequencing (WES) it allows researchers to achieve very high depths of coverage providing the increased sensitivity and accuracy needed for the analysis of low abundance cell free fetal DNA (cffDNA).
Cell3 Target is a novel target enrichment system developed by Nonacus for converting cell-free DNA or genomic DNA into libraries for next generation sequencing that has been validated to ensure confident detection and analysis for even the lowest of fetal fractions.

Reliable target enrichment for cffDNA and gDNA
We understand that our customers have different questions to answer so Cell3 Target has been developed for and validated on cffDNA and gDNA for a range of prenatal applications. Whether you are using amniocentesis or NIPT, you know you will be able to use the same method and panel design.
Quick and convenient workflow for cfDNA
Cell3 Target offers quick, Covaris-free, enzymatic shearing for genomic DNA samples and is available without enzymatic fragmentation reagents for cfDNA.
Quick and easy workflow takes less than 10 hours, with less than two hours hands-on time. Supports manual or automated preparation of 1–96 samples in a single batch 384 patient/sample indexes ensure that customers can use Cell3 Target on the smallest to the largest output sequencers. Suitable for use from 1 ng cfDNA or gDNA.

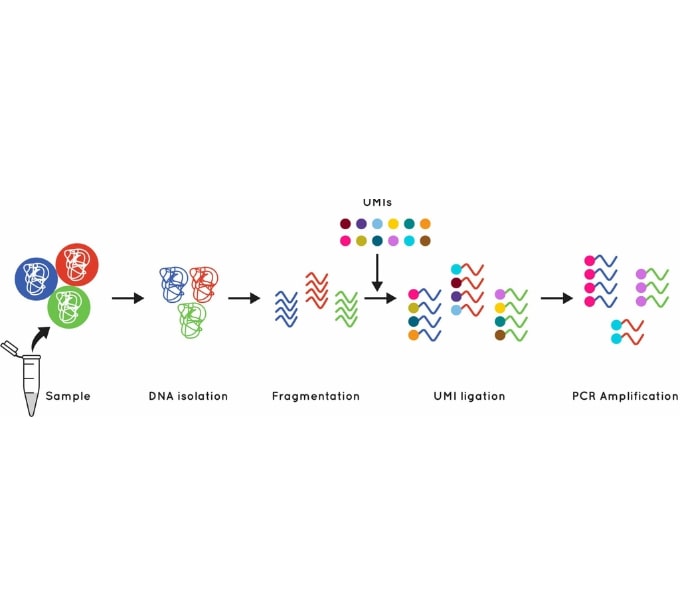
Figure 1: Using UMI’s to identify and quantify individual DNA molecules during library preparation increases sensitivity.
Ultra-sensitive NGS enrichment for NIPD
Cell3 Target enrichments incorporate error suppression technology, including unique molecular indexes (UMIs) and unique dual indexes (UDIs), which remove both PCR and sequencing errors and index hopping events. This error suppression technique, combined with our excellent uniformity of coverage, allows you to confidently and accurately call variants in even the lowest of fetal fractions. (and from as little as 1 ng cfDNA or gDNA input). We provide advice and provision of ready to go analysis scripts for error removal using UMIs.
Successful custom panel design for targeted NGS
Good design is key to a successful NGS panel ensuring adequate coverage and robust variant calling. It’s not always easy to achieve, especially the first time, but our Panel Design tool makes it simple and easy allowing you to optimize panels in minutes. Create designs instantly from scratch using uploaded BED files, gene lists or genomic coordinates, customize catalog products or add your own content onto one of our fixed panels.
- Easily merge, compare and share panels
- Get coverage data instantly and request a quote
- Our rapid manufacturing process means minimal time between design and delivery
Start your custom design now using the Panel Design Tool
Optimized sequencing performance for NIPD
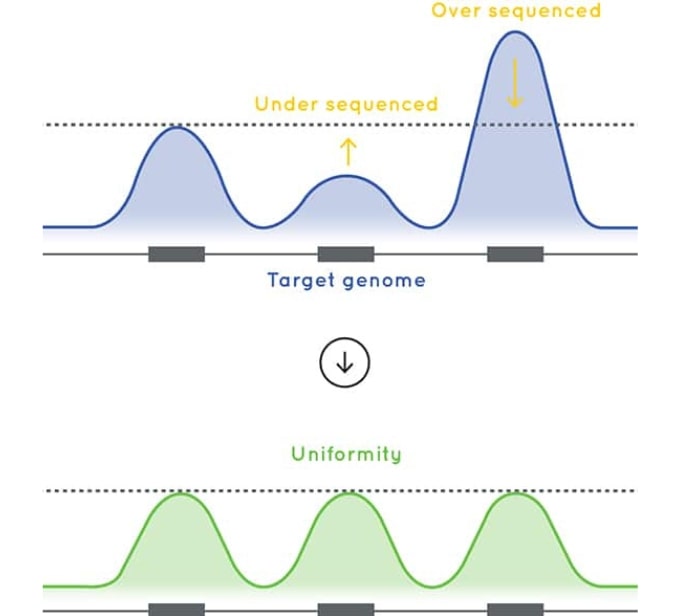
The baits used in Cell3 Target are designed to deliver excellent uniformity of coverage. By improving uniformity of coverage and reducing the number of low coverage exons, our Cell3 Target enrichment optimizes sequencing efficiency and sample capacity per sequencing run ultimately reducing your sequencing costs.
Improved on-target for small and large panels
Most capture target enrichment technologies suffer from a significant percentage of off target sequencing reads when targeting small regions of the genome. Cell3 Target has been developed so that whether you wish to sequence 1-1000 genes you will find lower off target and more uniform coverage when compared with alternative capture technologies.
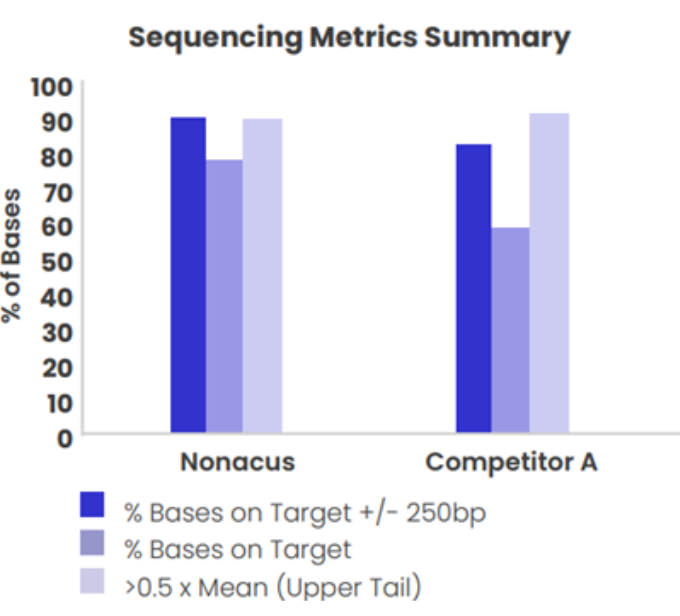
Figure 2: Coverage uniformity and % target capture for a 40 Kb panel designed using Cell3™ Target technology and Company (A) High Sensitivity enrichment kit. Targeted enrichment performed on genomic DNA samples and sequenced on the Illumina MiSeq.
For Research Use Only. Not for use in diagnostic procedures.
References
1. Barrett AN, Zimmermann BG, Wang D, Holloway A, Chitty LS. Implementing prenatal diagnosis based on cell-free fetal DNA: accurate identification of factors affecting fetal DNA yield. PloS One. 2011;6(10):e25202. Full article.
2. Nigam A, Saxena P, Prakash A, Acharya AS. Detection of fetal nucleic acid in maternal plasma: A novel noninvasive prenatal diagnostic technique. JIMSA. 2012;25(3):119-200. Full article.
See our customer publications
Cell3™ Target panels are available with one of two versions of our library preparation kits:
- Fragmentation: for use with gDNA (FF or FFPE)
- Non-Fragmentation: for use with cell-free DNA
| Panel Size - XS | 50-500 probes | |
| Frag | Non-Frag | |
| 48 Samples | NGS_C3C_XS_FR_48 | NGS_C3C_XS_NF_48 |
| 96 Samples | NGS_C3C_XS_FR_96_A/B/C/D* | NGS_C3C_XS_NF_96_A/B/C/D* |
| Panel Size - S | 501-1000 probes | |
| Frag | Non-Frag | |
| 48 Samples | NGS_C3C_S_FR_48 | NGS_C3C_S_NF_48 |
| 96 Samples | NGS_C3C_S_FR_96_A/B/C/D* | NGS_C3C_S_NF_96_A/B/C/D* |
| Panel Size - M | 1001-5000 probes | |
| Frag | Non-Frag | |
| 48 Samples | NGS_C3C_M_FR_48 | NGS_C3C_M_NF_48 |
| 96 Samples | NGS_C3C_M_FR_96_A/B/C/D* | NGS_C3C_M_NF_96_A/B/C/D* |
| Panel Size - L | 5001-7500 probes | |
| Frag | Non-Frag | |
| 48 Samples | NGS_C3C_L_FR_48 | NGS_C3C_L_NF_48 |
| 96 Samples | NGS_C3C_L_FR_96_A/B/C/D* | NGS_C3C_L_NF_96_A/B/C/D* |
| Panel Size - XL | 7501-10,000 probes | |
| Frag | Non-Frag | |
| 48 Samples | NGS_C3C_XL_FR_48 | NGS_C3C_XL_NF_48 |
| 96 Samples | NGS_C3C_XL_FR_96_A/B/C/D* | NGS_C3C_XL_NF_96_A/B/C/D* |
| Panel Size - XXL | 10,000-25,000 probes | |
| Frag | Non-Frag | |
| 48 Samples | NGS_C3C_XXL_FR_48 | NGS_C3C_XXL_NF_48 |
| 96 Samples | NGS_C3C_XXL_FR_96_A/B/C/D* | NGS_C3C_XXL_NF_96_A/B/C/D* |
* To provide flexibility in multiplexing samples, our 96-sample kits offer a choice in adapter plate:
A = Adapter plate with indexes 1-96
B = Adapter plate with indexes 97-192
C = Adapter plate with indexes 193-288
D = Adapter plate with indexes 289-384
